AcuPebble RE100 is a wearable device that fundamentally improves the measurement of respiratory biosignals. It provides more accurate data and enables the continuous monitoring of patients at home in clinical trials and post-market surveillance.
Clinical trials
Clean respiratory and cardiac signals and accurate physiological parameters to better understand and improve diagnosis methods and drug performances.
Post-market surveillance
Monitor the safety and performance of drugs or therapeutic devices after they are released on the market.

Accurate & remote data gathering
- Measure breathing and cardiac signals remotely.
- Clinically validated physiological parameters and biomarkers.
Easy for patients to use
- Non-invasive and wireless, patient can carry on with normal life.
- Does not require specialist to operate device or train the patient.
Safe and scalable solution
- Eliminates need for patient to visit healthcare sites.
- Easy to deploy and manage in large clinical trials.
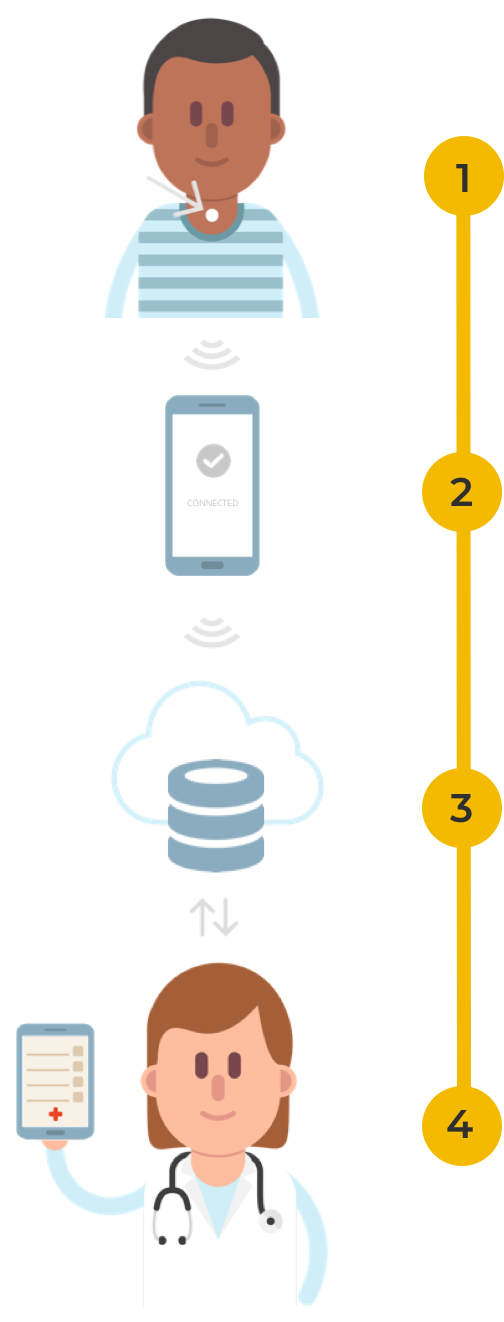
How does it work?
- The AcuPebble RE sensor is attached at the base of the neck to record the sounds generated by the patient’s respiratory and cardiac functions.
- The signals are transferred wirelessly to a mobile device, and then uploaded to a secure cloud platform.
- Proprietary algorithms automatically extract signal processing features and physiological parameters that can be used to carry out research of important respiratory and cardiac conditions including but not limited to OSA, COPD, Asthma and Coronaviruses.
- Researchers can access and analyse the data recorded remotely via a user-friendly web application.
Clinical research is the gathering of information to use in the development of safe and effective new approaches to healthcare, including clinical evidence to improve our understanding of certain medical conditions.
Current methods for the continuous monitoring of respiratory parameters are sub-optimal. They often require research subjects to remain in a controlled clinical environment, or are limited to spot checks.
AcuPebble RE100 addresses the problems of existing measuring devices, providing more accurate data and significantly lowering the average cost of clinical research by eliminating the need for stays in clinics.
Regulatory information

|
Acurable is ISO 13485 certified by MTIC InterCert SRL. |

|
Compliant with EU General Data Protection Regulation (GDPR). |