Clinically validated diagnosis
Proven Clinical Validation and Reliability.
- Validated sleep study result equivalent to ambulatory gold-standard (multi-channel polygraphy followed by manual specialist interpretation).
- Validated result based on AHI and ODI for both 3% and 4% desaturation criteria with high accuracy (94% PPV, 98% NPV).
- Proven usability of the device, with 100% of the patients in the trial able to complete the test without training.
Clinical Evidence
Multi-channel information
The system accurately extracts multi-channel information from 2 wireless devices: a neck sensor and a finger sensor to provide a complete sleep study report that includes oximetry information. (Number of channels: 8)
- SpO2
- Cardiac signal
- Activity (movement)
- Respiratory phases
- Airflow
- Snoring
- Pulse rate
- Body position
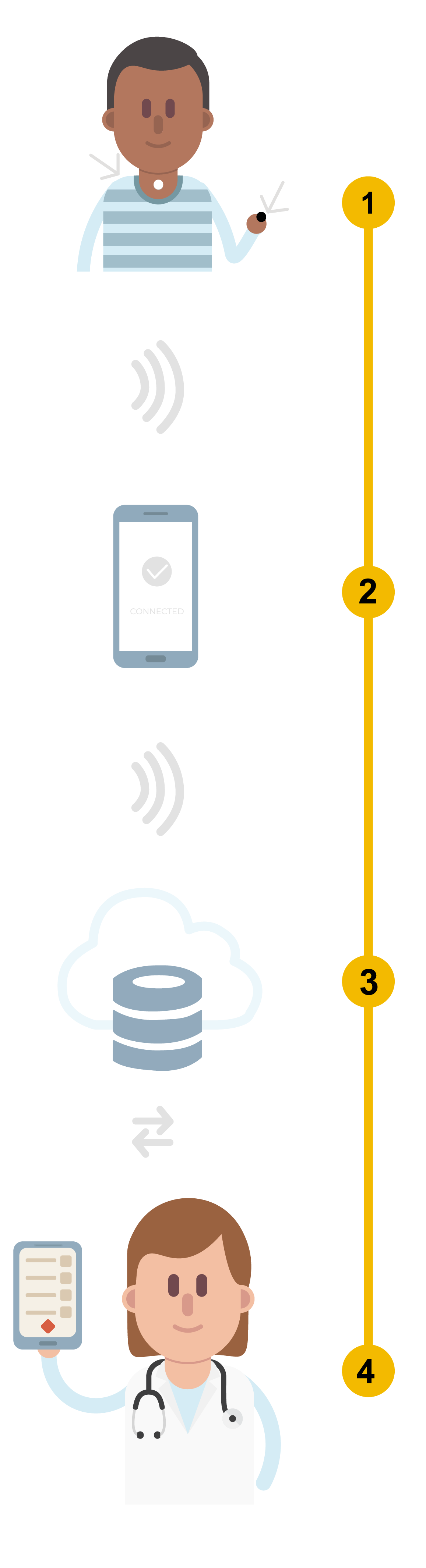
How does it work?
- AcuPebble Ox100 includes a sensor that is attached at the base of the neck to record sounds rich with information generated by the patient’s respiratory and cardiac functions and a finger oximeter to record the patient’s oxygen saturation level.
- The signals are transferred wirelessly to a mobile device, and then uploaded to a secure cloud platform.
- Sophisticated signal processing algorithms are applied to the signals to automatically extract the parameters used for sleep apnea diagnosis.
- Healthcare professionals get an automatically generated full diagnosis report within minutes, accessible via a user-friendly web application.
Patient-friendly design
All aspects of AcuPebble Ox100 have been designed with the patient in mind:
- Easy to use, as simple as peeling off an adhesive and putting it on.
- Non-invasive, allows patients to have a more natural sleep at home.
- No training required, avoids unnecessary trips to the clinic.
patient benefitsPatient benefits
Detailed sleep report
Detailed contents
-
Overall test result and severity
-
AHI obtained from flow reductions and 3% desaturation (as per current AASM criteria)
-
AHI obtained from flow reductions and 4% desaturation
-
ODI using 3% desaturation
-
ODI using 4% desaturation
-
All parameters obtained using estimation of sleep time
Additional information
-
Classification of apnea events
-
Cardiac feature analysis
Best alternative to Poligraphy
Reliable
-
Accurate diagnosis equivalent to CR-PG.
-
More natural sleep at home for the patient.
Saves time
-
No training required for patient (~1% failure rate).
-
Automated analysis (with option for manual review).
Preferred by patients
-
Easy to use with instructions in mobile app.
-
Comfortable to wear.
Helps improve Patient Care
Due to the ease of use, clinicians can implement new clinically useful pathways.
- Multi-night studies to assess the patient’s internight variability.
- Titration of therapeutic devices to achieve optimal adjustment.
- Monitoring of treatment to quantify patient evolution and improvement.